- Home
- Knowledge library
- How to detect herbicide resistance in arable weeds
How to detect herbicide resistance in arable weeds
The results of regular herbicide resistance tests (on seeds or plants) provide a strong indication of the unique resistance profiles present across each field. Find out how to sample and interpret test results to inform your long-term weed management strategies.
Weed management in the arable rotation
Introduction to herbicide resistance in arable weeds
General resistance-screening tips
- Test seed or plant samples regularly, especially if you suspect resistance is developing
- Aim to test for herbicide resistance at least once every two to three years
- Keep accurate field records of cropping, cultivation and herbicide use, as well as control achieved
- Ensure samples are representative of the field
- Resistance variation within an average black-grass patch is likely to be negligible
- Greater resistance variation usually occurs between patches – with even more variation across fields
- Keep a record of the level of weed infestation and the areas sampled (GPS reading or a sketched map)
- The best technique and timing varies with weed species
- Discuss sample collection and testing options with your adviser or crop protection supplier
|
Sampling unit |
Comments |
|
Patches |
One sample is likely to be representative of that patch |
|
Within fields |
Sample several patches across the field |
|
Between fields |
Be prepared to take several samples from each field |
|
Farms |
Never use results at one farm to predict those of another |
How to collect seed samples for testing
- Most tests use seeds from weeds that survive herbicide treatment, although some tests use plants
- Collect seeds from many plants in an area of about 100 m by 2–3 tramlines (unless the problem occurs in a distinct patch)
- Collect samples when most seeds are mature and 10–20% have shed
- Do not collect samples too early or too late, as this is likely to lead to samples with low viability
- Do not collect intact heads, as many seeds will be immature
- Allow seed to air-dry (away from sunlight) in a large shallow open container or paper sack for a few days
- Transfer samples to paper bags/envelopes for storage and transport (never store in polythene bags)
- Send samples to the testing centre as soon as possible
Herbicide resistance tests
Usually, resistance tests are based on herbicide treatment survivors. This bias means results are likely to exaggerate the level of resistance in the weed seed bank. However, it provides an indication of how populations are changing.
Glasshouse pot seed tests
A herbicide is applied to plants (typically at 2–3 leaf stage for post-emergence applications) established from weed seed samples, sown in pots. Plants are assessed 3–4 weeks later. The test is suitable for all weed species and herbicides.
Glasshouse pot plant tests
Surviving plants are collected from the field, transferred to pots (after trimming), allowed to regrow, sprayed, and assessed. The test is not suitable for pre-emergence herbicides.
Laboratory petri-dish seed assays
Seeds are germinated in herbicide solutions within petri-dishes. Seedling growth is recorded after 14 days. Although relatively quick, the test is not suitable for all weed species and herbicides.
Laboratory molecular leaf tests
Leaf samples are used in molecular tests to detect for mutations known to confer target site resistance (TSR). The tests are fast, although relatively high cost.
Resistance in-season quick plant test (RISQ)
Small-to-early tillered plants are collected from the field and placed in agar solution containing a herbicide dose. Root growth is measured after 14 to 21 days. This rapid test works best on grass-weed species and is relatively high cost.
Field-based plant assay
Leaf samples are analysed for the presence of a protein that regulates enhanced metabolism resistance (EMR). This test is very fast and relatively high cost.
Radio-labelled laboratory plant tests
The rate of metabolism of radio-labelled herbicide in leaves is measured. It is a very fast and high-cost test. Ideally, test results should include:
- Results from susceptible and resistant reference populations, ideally with resistance mechanisms known
- Photographic records of the tests
- Information on the assessment method and basis on which resistance is assigned, especially when populations show marginal or partial resistance.
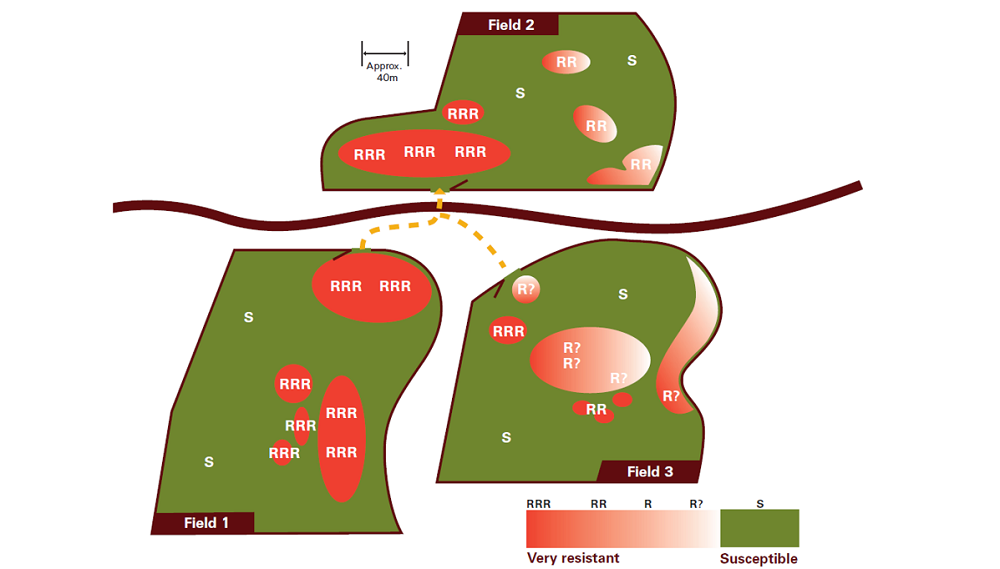
UK resistance-rating ‘R’ system
 AHDB
AHDB
- RRR – resistance highly likely to reduce herbicide performance
- RR – resistance likely to reduce herbicide performance
- R? – early indications of resistance development, possibly reducing herbicide performance
- S – susceptible, no effect on herbicide performance
An example of how resistance spreads across fields is provided in the illustration, above.
Note: This information is based on the WRAG publication entitled: Maximising the benefits of herbicide resistance testing.
.jpg)
Topics:
Sectors:
Tags:

