- Home
- Knowledge library
- Abortion vaccines in sheep
Abortion vaccines in sheep
Vaccines are available for the main infectious causes of abortion in sheep. It is possible to control enzootic abortion without antibiotics by using effective vaccination strategies.
The three main infectious causes of abortion, which have been consistent over the last five years, are:
- Enzootic abortion of ewes (EAE) caused by the bacteria Chlamydia abortus
- Toxoplasmosis (infection by the Toxoplasma gondii parasite)
- Campylobacter spp. bacterial infection
View APHA's Sheep Disease Surveillance Dashboard
Although the epidemiology of these three infections is very different, any one of them can cause outbreaks of abortion in flocks. In addition, these pathogens are zoonotic and can cause illness and abortion in humans.
The control of enzootic abortion was identified as one of three hotspot areas for the reduction of antibiotics in the sheep industry in the RUMA Targets Task Force Report 2020.
Alongside vaccination, good biosecurity measures are essential tools to protect flocks against enzootic abortion.
An abortion rate above 2% in a flock warrants investigation as it may be due to an infectious cause.
The cost per lamb lost by abortion is estimated to be at least £85 (Mearns, 2007) with vaccination providing a cost-effective measure to protect against enzootic abortion and toxoplasmosis.
Vaccines which protect against Campylobacter spp are not authorised for use in the UK without a veterinary special import certificate.
Effective vaccines against EAE and toxoplasmosis are authorised for use.
The live vaccines listed below must be handled with care, must not be handled by pregnant women, and cannot be given to pregnant animals.
- Enzovax is a live vaccine for the active immunisation of breeding female sheep against C. abortus infection
- Cevac® Chlamydia is a live vaccine for the active immunisation of breeding female sheep against C. abortus infection
- Inmeva Suspension For Injection is an inactivated vaccine for the active immunisation of breeding female sheep against C. abortus infection (and Salmonella Abortusovis infection although this bacteria is not currently present in the UK). It can be administered in pregnant sheep up to the last month of gestation, which can be useful in the face of an enzootic abortion outbreak (see datasheet for full instructions)
- Toxovax® is a live vaccine containing Toxoplasma gondii parasites
Assumptions
Numerator
The number of doses of vaccine administered has been calculated by multiplying the number of packs sold by the number of doses per pack. The Inmeva vaccine requires a two-dose primary course.
Denominator
The common industry recommendation is to vaccinate all breeding females before the first joining with the ram.
Although revaccination may be advisable after three to four years, it is not thought that a considerable amount of vaccine is used in this way.
The total number of vaccine doses that would be required to protect the national UK flock from EAE and toxoplasmosis has been estimated based on the assumption that all ewes intended for first-time breeding in June should receive a dose of vaccine (or a two-dose primary course for Inmeva).
It is accepted that this denominator is probably an underestimation of the actual number of ewes that should receive vaccination, but it was chosen for both simplicity and repeatability.
Vaccination uptake
Enzootic abortion vaccination
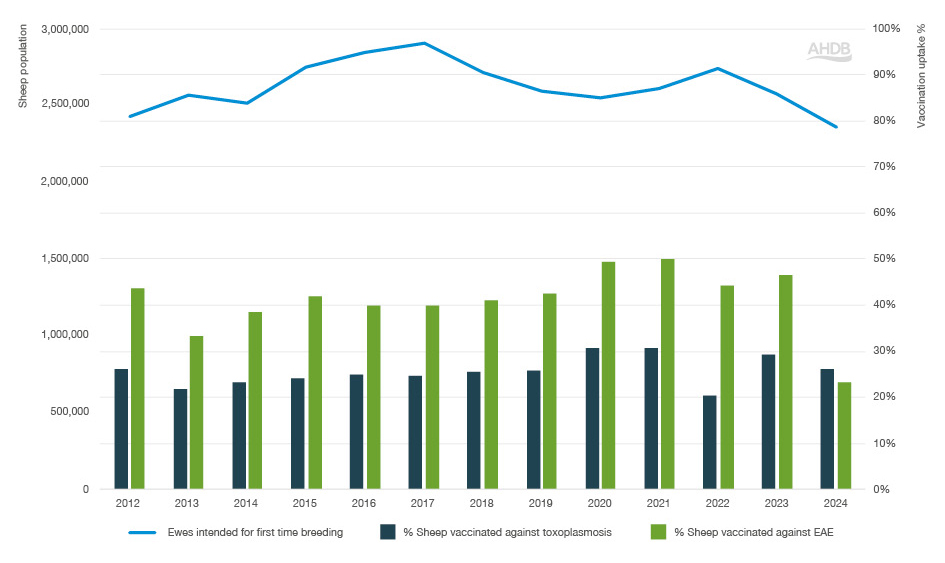
From 2013 to 2021 there was a steady increase in estimated uptake of vaccination against EAE, from one in three ewes to half of ewes vaccinated against EAE.
In 2022, this dropped to 44%, before rising to 46% in 2023. However, in 2024, vaccine uptake fell to 23% which is the lowest recorded uptake since the report started in 2012. This is likely due to the severe EAE vaccine shortages seen in 2024.
Toxoplasmosis vaccination
In 2023, 29% of breeding ewes and ewes intended for first-time breeding were estimated to be vaccinated against toxoplasmosis. In 2024, this dropped to an estimated uptake of 26%.
The manufacture of these live abortion vaccines is known to be complicated, and the timing for administration is generally concentrated over a short period, just before the breeding season.
In recent years, there have been several occasions when whole batches of vaccines have failed quality control and supply has been limited at key times of the year.
These manufacturing issues are likely to affect both supply and uptake.
Due to the severity of veterinary vaccine supply shortages over the last few years, in September 2025, the Veterinary Medicines Directorate (VMD) published a Statement of Intent outlining a framework to tackle this issue.
Figure 1. Percentage of sheep vaccinated against toxoplasmosis and EAE