- Home
- Knowledge library
- Rhizoctonia solani symptoms and risk in oilseed rape
Rhizoctonia solani symptoms and risk in oilseed rape
Found in soils and crops worldwide, this pathogen is associated with major establishment losses. With no chemical control options for oilseed rape, a good understanding of the pathogen is required to target non-chemical management approaches.
Symptoms of rhizoctonia
Rhizoctonia solani is a common soilborne crop pathogen that can infect oilseed rape, in addition to broccoli, cabbage, cauliflower, peas, beans, radish, wheat, barley, potatoes and many weed species.
Crops are most susceptible during the seedling stage. Infection can result in seed decay and pre- and post-germination damping off. Ultimately, seedling death results in poor emergence/establishment.
On surviving plants, the pathogen may reduce taproot length and decrease the number of lateral roots. Severe infections can cause a large reduction in the total root surface area.
 University of Nottingham
University of Nottingham
Figure 1. Oilseed rape infected with R. solani AG2-1 resulted in the death of young seedlings and patchy emergence.
Early symptoms include thinning and elongation of stems (known as ‘wirestem’) and severing of roots at the point of infection (known as ‘spear heading/tipping’). Such symptoms are not easy to diagnose, as the fragile seedlings often break at the infection point during sample collection.
Symptoms on mature plants include brown, necrotic lesions, which may girdle the root or stem circumference. Infected plants may also exhibit delayed flowering. Other soilborne pathogens cause similar symptoms, making accurate diagnosis a challenge.
How much damage can rhizoctonia cause?
In oilseed rape, the pathogen is associated with establishment losses of 17–65%. Where plant numbers are reduced to below the optimum (25–35 plants/m2), yield loss can occur. However, yield reduction can also occur if mature plants suffer significant root rotting.
 University of Nottingham
University of Nottingham
Figure 2. Oilseed rape infected with R. solani AG2-1 showed delayed flowering compared to the controls. Many of these plots also featured significant bare patches.
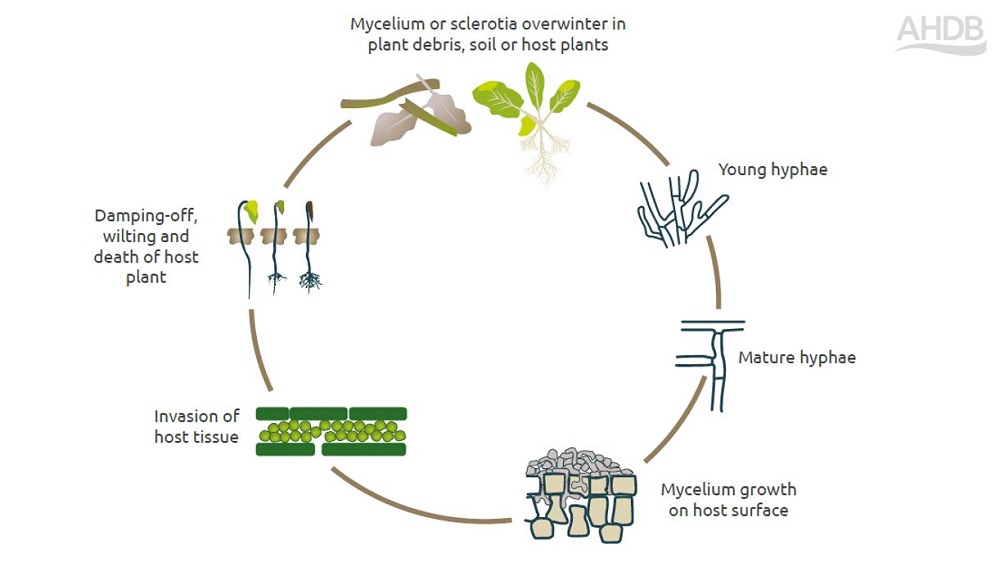
Life cycle of rhizoctonia
Rhizoctonia solani is a species complex that comprises 14 anastomosis groups (AGs). Pathogenic isolates are allocated to a group based on their ability to fuse (anastomose) with each other. AGs 2-1, 4 and 8 are most pathogenic to oilseed rape. AG 2-1 isolates are prevalent in soils in England.
In the soil, the pathogen survives as resting structures called sclerotia (made of compacted mycelia) or as hyphae on crop debris or seeds. In the absence of a host, R. solani can survive on crop debris for long periods, and on crop volunteers and weeds.
Oilseed rape releases chemicals (exudates) that stimulate the production of runner hyphae from germinated sclerotia or overwintering mycelia. Once the host is reached, the pathogen forms an infection peg and produces digestive enzymes to degrade plant cell walls and infect the plant. Hosts are most susceptible during emergence and seedling development.
Older mycelia form sclerotia on infected tissue. Where tissue is left in/on the soil (e.g. crop debris), it can become a source of infection for following crops. When susceptible crops are grown in close rotations, pathogen inoculum/pressure builds.
 AHDB
AHDB
Management of rhizoctonia
A strategy based on integrated control methods is the most effective.
Reduce soil inoculum density
- Include non-host crops or extend the break between the most susceptible hosts
- Grow oilseed rape no more than 1 in 4 in the rotation
- Remove or destroy volunteer plants and weeds that harbour the pathogen
- Plough or deep till down to 8–10 cm to disturb hyphal networks and reduce inoculum*
*Note a careful combination of biology (e.g. roots and worms) and metal (e.g. plough) is the most efficient way to manage soil. Read our guidance to strike the right balance in your fields.
Arable soil management: Cultivation and crop establishment
Reduce opportunities for infection
- No oilseed rape varieties carry resistance to solani AG2-1
- Select varieties and seed stocks with high seed viability and vigour
- Avoid deep sowing (below 6 cm) as this can slow time to emergence
- Optimise seedbed conditions – dry soils are associated with increased disease severity, as is warmer weather
- Increased soil water during emergence slows fungal spread and host colonisation
Increase crop resilience
- Increase seed rates to help offset plant losses, especially for later sowings in higher-risk situations
A note on seed treatments
Thiram-based seed treatments are no longer permitted (since 30 January 2020). Research trials have established the potential of active ingredients of the class of succinate dehydrogenase inhibitors (SDHIs) but these are not currently authorised for use in oilseed rape.
Further information
This information has been produced as part of one of twenty-four innovative agri-tech projects. These were awarded £16 million through the government’s Agri-Tech Catalyst.
Find out more about our rhizoctonia research
Topics:
Sectors:
Tags:

