- Home
- Knowledge library
- Pneumonia vaccines in cattle
Pneumonia vaccines in cattle
An important part of the herd health plan is an effective vaccination strategy for common causes of pneumonia. To prevent respiratory disease, good management, appropriate building design and ventilation are also important.
Respiratory disease occurs when bacterial or viral agents (sometimes both simultaneously) are combined with poor air quality and ventilation, poor husbandry or stress. Calves are more susceptible than adult cattle.
Financial losses result from mortality, extra labour and treatment costs, but the greatest loss is from weight loss during illness and recovery.
Vaccination is a cost effective tool to aid in the prevention of pneumonia.
Seventeen vaccines for pneumonia are licensed in the UK (updated October 2025):
- Bovalto Respi 3 contains inactivated M. haemolytica A1, inactivated Bovine respiratory syncytial and Parainfluenza 3 viruses
- Bovalto Respi 4 contains inactivated M. haemolytica A1, inactivated Bovine respiratory syncytial, Parainfluenza 3 and BVD viruses
- Bovalto Respi Intranasal contains modified live Bovine respiratory syncytial virus and Parainfluenza 3 virus
- Bovilis Bovipast RSP contains inactivated M. haemolytica A1, inactivated Bovine respiratory syncytial and Parainfluenza 3 viruses
- Bovilis® INtranasal RSP™ Live contains modified live Bovine respiratory syncytial virus and Parainfluenza 3 virus
- Bovilis ® Nasalgen-C contains modified live bovine coronavirus
- Hiprabovis SOMNI/Lkt contains inactivated M. haemolytica leukotoxoid and inactivated Histophilus somni
- NASYM contains modified live Bovine respiratory syncytial virus
- Pneumovac contains inactivated M. haemolytica A1, and inactivated Bovine respiratory syncytial and Parainfluenza 3 viruses
- Pneumovac Plus contains inactivated M. haemolytica A1, inactivated Bovine respiratory syncytial, Parainfluenza 3 viruses and live BVD virus
- Rispoval 2 contains modified live Bovine respiratory syncytial virus and Parainfluenza 3 virus
- Rispoval 3 contains modified live Bovine respiratory syncytial and Parainfluenza 3 viruses and inactivated BVD virus
- Rispoval 4 contains modified live Bovine respiratory syncytial and Parainfluenza 3 viruses, and inactivated BVD and Bovine herpesvirus type 1 (BVH-1) viruses
- Rispoval® Pasteurella contains inactivated M. haemolytica
- Rispoval RS contains modified live Bovine respiratory syncytial virus
- Rispoval RS+PI3 Intranasal contains modified live Bovine respiratory syncytial and Parainfluenza 3 viruses.
- Protivity contains modified live Mycoplasma bovis
Vaccines for Infectious Bovine Rhinotracheitis (IBR) containing only Bovine herpesvirus type 1 (BHV-1) have not been included in the estimated uptake of vaccines targeted at calf pneumonia.
The uptake of vaccines for IBR has been estimated separately.
Assumptions
Numerator
The number of doses of vaccine administered has been calculated by multiplying the number of packs sold by the number of doses per pack.
The recommended primary vaccination course for these vaccines was used to estimate the number of cattle vaccinated in each calendar year.
Denominator
With these vaccines, the common industry practice is only to vaccinate animals in the first year of life. It was assumed that only animals under one year of age were vaccinated; the total population of cattle under one year of age was used as the denominator for the target population.
Vaccination uptake
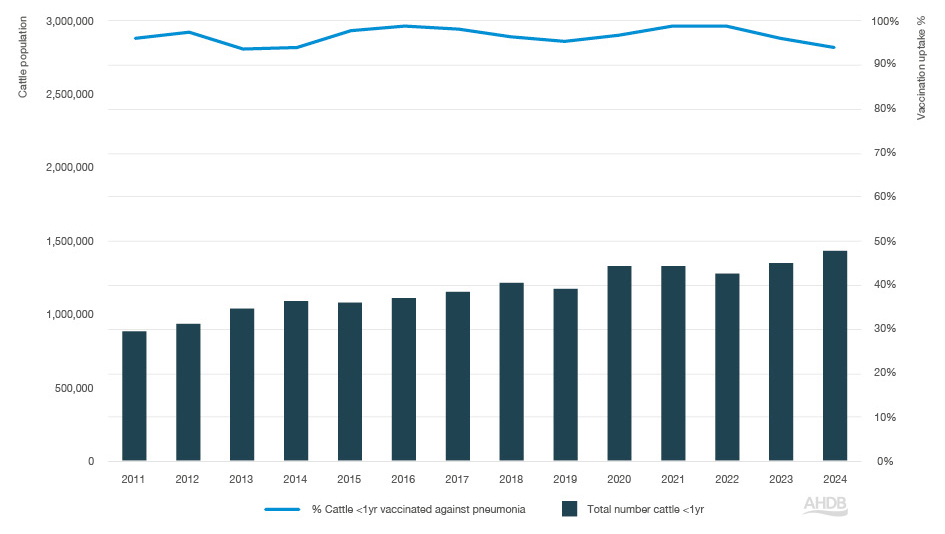
Pneumonia vaccination has steadily increased since 2011 and was estimated at 48% in 2024.
Figure 1. Percentage of cattle vaccinated against pneumonia